b.Bone Clinical Evidence

Clinical Evidence
b.Bone is a CE – marked medical device with pre-clinical and clinical data to demonstrate the product’s safety and performance, and support the benefits it provides, namely its regenerative and mechanical capabilities, previously demonstrated in-vitro (1). Clinical Evidence is collected through Clinical Studies and other Post-Market Surveillance activities.
GreenBone is committed to generating high-quality clinical evidence to support our products. Clinical evidence is collected from the extensive and practical experience from international Key Opinion Leaders and their multi-disciplinary teams.
- Kon E, Salamanna F, Filardo G, Di Matteo B, Shabshin N, Shani J, Fini M, Perdisa F, Parrilli A, Sprio S, Ruffini A, Marcacci M, Tampieri A. Bone Regeneration in Load-Bearing Segmental Defects, Guided by Biomorphic, Hierarchically Structured Apatitic Scaffold. Front Bioeng Biotechnol. 2021 Sep 27;9:734486
An overview of b.Bone key clinical and safety data
GreenBone conducts and promotes clinical research to introduce our technology through studies demonstrating safety and performance, and to provide long term clinical outcomes data.
GreenBRIC study
Study Title
First in-human study “Multi-centre, national open-labels, single-arm study to evaluate the safety and performance of b.Bone for iliac crest reconstruction following bone graft harvesting for pelvic fusion.”
Clinical Evidence
Complete bone formation, remodelling and union at 1 year. Radiologically, the bone healing process observed was indicative of full device integration and bone restoration within the defect area at 6 months in all patients (100%).
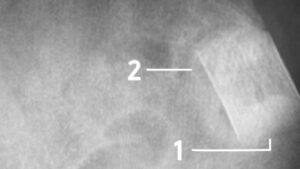
Fig 1: Magnification at 6 months follow up demonstrates complete integration of b.Bone (1) with mineralization similar to the pelvic bone texture (2). Courtesy of prof. P.V. Giannoudis
- Favourable outcomes were reported for pain (VAS score) and quality of life (EQ-5D-5L score). The average pain decreased from severe to mild at 1 year. The average EQ-5D-5L score improved of 30% at 1 year. Bone was proved to be user-friendly and easy to implant
- The bone defect area in the iliac crest of the pelvis was well covered and the local anatomy restored with no difficulty. The first radiological signs of healing were observed at 60 days following implantation
- The results from this clinical investigation confirmed that the use of b.Bone in iliac crest reconstruction following bone graft harvesting, prevents donor site morbidity (3) .
2. Internal data report
LongBone study
Study Title
“Multi-centre, international, open-label, single-arm study to evaluate the safety and performance of b.Bone surgical repair of long bone defects.”
Clinical Evidence
- Average bone gap length: 2.3 cm;
- Type of damage:
- High-energy traumatic event (61.1%);
- Treatment of complex bone non-union (38.9%);
- Site of injury:
- Humerus (16.7%);
- Radius (16.7%);
- Ulna (22.2%);
- Femur (22.2%);
- Tibia (22.2%).
- b.Bone proved to be safe and easy to use
- The results achieved are comparable to those reported in the literature for the treatment of large diaphyseal defects using different surgical techniques (2) .
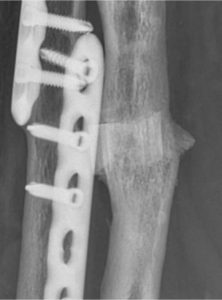
Fig.2 Magnification at 19 months follow up in radius reconstruction. Courtesy of prof. A. Kirienko
3. Feltri, P., Solaro, L., Di Martino, A. et al.Union, complication, reintervention and failure rates of surgical techniques for large diaphyseal defects: a systematic review and meta-analysis. Sci Rep 12, 9098 (2022).
The post market OSs-IRIS
Study Title
“A post-market clinical follow up investigation to confirm the performance and safety of the bone substitute b.Bone in extremities and pelvis”.
The post market OSs-IRIS study will gather clinical data about the performance and safety of b.Bone on patients who require orthopaedic interventions in the pelvis and in the extremities. It has been designed according to MDR 2017/745 requirements.
